This article is about the fluoride ion. For a review of fluorine compounds, see Compounds of fluorine. For the fluoride additive used in toothpaste, see Fluoride therapy. For the 2015 French film, see Floride (film).
|
|
|||
| Names | |||
|---|---|---|---|
| IUPAC name
Fluoride[1]
|
|||
| Identifiers | |||
| 16984-48-8 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:17051 | ||
| ChEMBL | ChEMBL1362 |
||
| ChemSpider | 26214 |
||
| 14905 | |||
| KEGG | C00742 |
||
| MeSH | Fluoride | ||
| PubChem | 28179 | ||
| Properties | |||
| F− | |||
| Molar mass | 19.00 g·mol−1 | ||
| Thermochemistry | |||
|
Std molar
entropy (S |
145.58 J/mol K (gaseous)[2] | ||
|
Std enthalpy of
formation (ΔfH |
−333 kJ mol−1 | ||
| Related compounds | |||
|
Other anions
|
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
Contents
Nomenclature
The systematic name fluoride, the valid IUPAC name, is determined according to the additive nomenclature. However, the name fluoride is also used in compositional IUPAC nomenclature which does not take the nature of bonding involved into account. Examples of such naming are sulfur hexafluoride and beryllium fluoride, which contain no fluoride ions whatsoever, although they do contain fluorine atoms.Fluoride is also used non-systematically, to describe compounds which releases hydrogen fluoride upon acidification, or a compound that otherwise incorporates fluorine in some form, such as methyl fluoride and fluorosilicic acid. Hydrogen fluoride is itself an example of a non-systematic name of this nature. However, it is also a trivial name, and the preferred IUPAC name for fluorane.
Occurrence
Fluorite crystals
Seawater fluoride levels are usually in the range of 0.86 to 1.4 mg/L, and average 1.1 mg/L[5] (milligrams per litre or ppm - parts per million of fluorine by weight compared with water - effectively interchangeable terms). For comparison, chloride concentration in seawater is about 19 g/L (19000 ppm). The low concentration of fluoride reflects the insolubility of the alkaline earth fluorides, e.g., CaF2.
Fluoride is present naturally in low concentration when drinking water and foods are based on surface (rain/river) water... such water supplies generally contain between 0.01–0.3 ppm.[6] Groundwater (well water) concentrations vary even more, depending on the composition of the local ground; for example under 0.05 ppm in parts of Canada to 2800 mg/litre, although rarely exceeeds 10 mg/litre[7]
- In some locations, the drinking water contains dangerously high levels of fluoride, leading to serious health problems.
- 50 million people receive water from water supplies that have close to the "optimal level".[8]
- In other locations the level of fluoride is very low, sometimes leading to fluoridation of public water supplies to bring the level to around 0.7-1.2 ppm.
Chemical properties
Basicity
Fluoride can act as a base. It can combine with a proton (H+):- F− + H+ → HF
In aqueous solution, fluoride has a pKb value of 10.8. It is therefore a weak base, and tends to remain as the fluoride ion rather than generating a substantial amount of hydrogen fluoride. That is, the following equilibrium favours the left-hand side in water:
- F− + H2O
HF + HO−
Structure of fluoride salts
Salts containing fluoride are numerous and adopt myriad structures. Typically the fluoride anion is surrounded by four or six cations, as is typical for other halides. Sodium fluoride and sodium chloride adopt the same structure. For compounds containing more than one fluoride per cation, the structures often deviate from those of the chlorides, as illustrated by the main fluoride mineral fluorite (CaF2) where the Ca2+ ions are surrounded by eight F− centers. In CaCl2, each Ca2+ ion is surrounded by six Cl− centers.Inorganic chemistry
Upon treatment with a standard acid, fluoride salts convert to hydrogen fluoride and metal salts. With strong acids, it can be doubly protonated to give H2F+. Oxidation of fluoride gives fluorine. Solutions of inorganic fluorides in water contain F− and bifluoride HF−
2.[12] Few inorganic fluorides are soluble in water without undergoing significant hydrolysis. In terms of its reactivity, fluoride differs significantly from chloride and other halides, and is more strongly solvated in protic solvents due to its smaller radius/charge ratio. Its closest chemical relative is hydroxide.[citation needed] When relatively unsolvated, for example in nonprotic solvents, fluoride anions are called "naked". Naked fluoride is a very strong Lewis base,[13] it is easily reacted with Lewis acids, forming strong adducts. Fluoride is susceptible to extreme ultraviolet radiation, ejecting an electron to become highly reactive atomic fluorine. It has a standard electrode potential of 2.87 volts.[citation needed]
Biochemistry
At physiological pHs, hydrogen fluoride is usually fully ionised to fluoride. In biochemistry, fluoride and hydrogen fluoride are equivalent. Fluorine, in the form of fluoride, is considered to be a micronutrient for human health, necessary to prevent dental cavities, and to promote healthy bone growth.[14] The tea plant (Camellia sinensis L.) is a known accumulator of fluorine compounds, released upon forming infusions such as the common beverage. The fluorine compounds decompose into products including fluoride ions. Fluoride is the most bioavailable form of fluorine, and as such, tea is potentially a vehicle for fluoride dosing.[15] Approximately, fifty percent of absorbed fluoride is excreted renally with a twenty-four-hour period. The remainder can be retained in the oral cavity, and lower digestive tract. Fasting dramatically increases the rate of fluoride absorption to near hundred percent, from a sixty to eighty percent when taken with food.[15] Per a 2013 study, it was found that consumption of one litre of tea a day, can potentially supply the daily recommended intake of 4 mg per day. Some lower quality brands can supply up to a 120 percent of this amount. Fasting can increase this to 150 percent. The study indicates that tea drinking communities are at an increased risk of dental and skeletal fluorosis, in the case where water fluoridation is in effect.[15] Fluoride ion in low doses in the mouth reduces tooth decay.[16] For this reason, it is used in toothpaste and water fluoridation. At much higher doses and frequent exposure, fluoride causes health complications and can be toxic.Applications
Fluoride salts and hydrofluoric acid are the main fluorides of industrial value. Compounds with C-F bonds fall into the realm of organofluorine chemistry. The main uses of fluoride, in terms of volume, are in the production of cryolite, Na3AlF6. It is used in aluminium smelting. Formerly, it was mined, but now it is derived from hydrogen fluoride. Fluorite is used on a large scale to separate slag in steel-making. Mined fluorite (CaF2) is a commodity chemical used in steel-making.Hydrofluoric acid and its anhydrous form, hydrogen fluoride, is also used in the production of fluorocarbons. Hydrofluoric acid has a variety of specialized applications, including its ability to dissolve glass.[4]
Cavity prevention
Main articles: Fluoride therapy and Water fluoridation
Fluoride is sold in tablets for cavity prevention.
Biochemical reagent
Fluoride salts are commonly used in biological assay processing to inhibit the activity of phosphatases, such as serine/threonine phosphatases.[23] Fluoride mimics the nucleophilic hydroxide ion in these enzymes' active sites.[24] Beryllium fluoride and aluminium fluoride are also used as phosphatase inhibitors, since these compounds are structural mimics of the phosphate group and can act as analogues of the transition state of the reaction.[25][26]Estimated daily intake
Daily intakes of fluoride can vary significantly according to the various sources of exposure. Values ranging from 0.46 to 3.6–5.4 mg/day have been reported in several studies (IPCS, 1984).[14] In areas where water is fluoridated this can be expected to be a significant source of fluoride, however fluoride is also naturally present in huge range of foods, in a wide range of concentrations.[27] The maximum safe daily consumption of fluoride is 10 mg for an adult.| Food/Drink | Fluoride (mg per 100 g) |
Portion | Fluoride (mg per portion) |
|---|---|---|---|
| Black tea (brewed) | 0.373 | 1 cup, 240 g (8 fl oz) | 0.884 |
| Raisins, seedless | 0.234 | small box, 43 g (1.5 oz) | 0.033 |
| Table wine | 0.153 | Bottle, 750 ml (26.4 fl oz) | 1.150 |
| Municipal tap-water, (Fluoridated) |
0.081 | Recommended daily intake, 3 litres (0.79 US gal) |
2.433 |
| Baked potatoes, Russet | 0.045 | Medium potato, 140 g (0.3 lb) | 0.078 |
| Lamb | 0.032 | Chop, 170 g (6 oz) | 0.054 |
| Carrots | 0.003 | 1 large carrot, 72 g (2.5 oz) | 0.002 |
Safety
Main article: Fluoride toxicity
Ingestion
According to the U.S. Department of Agriculture, the Dietary Reference Intakes, which is the "highest level of daily nutrient intake that is likely to pose no risk of adverse health effects" specify 10 mg/day for most people, corresponding to 10 L of fluoridated water with no risk. For infants and young children the values are smaller, ranging from 0.7 mg/d for infants to 2.2 mg/d.[28] Water and food sources of fluoride include community water fluoridation, seafood, tea, and gelatin.[29]Soluble fluoride salts, of which sodium fluoride is the most common, are toxic, and have resulted in both accidental and self-inflicted deaths from acute poisoning.[4] The lethal dose for most adult humans is estimated at 5 to 10 g (which is equivalent to 32 to 64 mg/kg elemental fluoride/kg body weight).[30][31][32] A case of a fatal poisoning of an adult with 4 grams of sodium fluoride is documented,[33] and a dose of 120 g sodium fluoride has been survived.[34] For sodium fluorosilicate (Na2SiF6), the median lethal dose (LD50) orally in rats is 0.125 g/kg, corresponding to 12.5 g for a 100 kg adult.[35]
The fatal period ranges from 5 min to 12 hours.[33] The mechanism of toxicity involves the combination of the fluoride anion with the calcium ions in the blood to form insoluble calcium fluoride, resulting in hypocalcemia; calcium is indispensable for the function of the nervous system, and the condition can be fatal.
Treatment may involve oral administration of dilute calcium hydroxide or calcium chloride to prevent further absorption, and injection of calcium gluconate to increase the calcium levels in the blood.[33] Hydrogen fluoride is more dangerous than salts such as NaF because it is corrosive and volatile, and can result in fatal exposure through inhalation or upon contact with the skin; calcium gluconate gel is the usual antidote.[36]
In the higher doses used to treat osteoporosis, sodium fluoride can cause pain in the legs and incomplete stress fractures when the doses are too high; it also irritates the stomach, sometimes so severely as to cause ulcers. Slow-release and enteric-coated versions of sodium fluoride do not have gastric side effects in any significant way, and have milder and less frequent complications in the bones.[37] In the lower doses used for water fluoridation, the only clear adverse effect is dental fluorosis, which can alter the appearance of children's teeth during tooth development; this is mostly mild and is unlikely to represent any real effect on aesthetic appearance or on public health.[38] Fluoride was known to enhance the measurement of bone mineral density at the lumbar spine, but it was not effective for vertebral fractures and provoked more non vertebral fractures.[39]
A popular urban myth claims that the Nazis used fluoride in concentration camps, but there is no historical evidence to prove this claim.[40]
In areas that have naturally occurring high levels of fluoride in groundwater which is used for drinking water, both dental and skeletal fluorosis can be prevalent and severe.[41]
Hazard maps for fluoride in groundwater
Around one-third of the world’s population drinks water from groundwater resources. Of this, about 10 percent, approximately 300 million people, obtains water from groundwater resources that are heavily polluted with arsenic or fluoride.[42] These trace elements derive mainly from minerals.[43] Maps are available of locations of potential problematic wells.[44]Topical
Concentrated fluoride solutions are corrosive.[45] Gloves made of nitrile rubber are worn when handling fluoride compounds. The hazards of solutions of fluoride salts depend on the concentration. In the presence of strong acids, fluoride salts release hydrogen fluoride, which is corrosive, especially toward glass.[4]Other derivatives
Organic and inorganic anions are produced from fluoride, including:- Bifluoride used as an etchant for glass.[46]
- Tetrafluoroberyllate
- Hexafluoroplatinate
- Tetrafluoroborate used in organometallic synthesis.
- Hexafluorophosphate used as an electrolyte in commercial secondary batteries.
- Trifluoromethanesulfonate
See also
- Fluoride deficiency
- Fluoride selective electrode
- Fluoride therapy
- 19F NMR spectroscopy
- Sodium monofluorophosphate
References
|chapter= ignored (help)External links
| Wikimedia Commons has media related to Fluorides. |
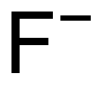


ليست هناك تعليقات:
إرسال تعليق